
The calcitic layers of the eggshells of archosaurs (including crocodilians and birds) and turtles are composed of distinctive crystalline structures known as eggshell units. Those growing from the shell membrane are called primary eggshell units (PEUs), while those forming within the calcitic layer are called secondary eggshell units (SEUs). Although rare in modern bird eggs, SEUs are common in dinosaur eggs. Due to the lack of in-depth research on this structure, however, scientists are uncertain whether they are biogenic or abiogenic in origin.
To tackle this issue, an international research team led by Dr. ZHANG Shukang and postdoctoral researcher Dr. CHOI Seung from the Institute of Vertebrate Paleontology and Paleoanthropology (IVPP) of the Chinese Academy of Sciences, has conducted a comprehensive study of SEUs in dinosaur eggshells using techniques including electron backscatter diffraction (EBSD), polarized light microscopy (PLM), and scanning electron microscopy (SEM). For comparison, they examined some eggshells of modern birds, turtles, and crocodiles.
The study revealed that the crystallographic characteristics of dinosaur egg SEUs were almost identical to those of PEUs. Notably, they also matched the crystallographic characteristics of the SEUs in modern turtle and crocodile eggshells.
Additionally, the SEUs in dinosaur eggs exhibited numerous grooves and vesicles, similar to those in modern bird eggshell units. These grooves and vesicles were interpreted as remnants of spaces left by the degradation of organic matrix fibers during fossilization. These findings strongly suggest that the SEUs in dinosaur eggs are biogenic structures.
Furthermore, in some dinosaur eggshells with well-developed pore canals, the researchers discovered that the SEUs either overlapped the PEUs or grew inside the pore canals. Although the growth of these SEUs was undisturbed, the c-axes of their calcite crystals still extended parallel to the growth direction of the eggshell. This phenomenon challenges the “competition hypothesis” derived from modern bird eggshells, and it reveals that the c-axis orientation of eggshell units is likely regulated by organic matrix fibers rather than the result of the competitive growth between adjacent calcite crystals.
Notably, when it comes to evolutionary dynamics, the researchers discovered that SEUs were present in the eggshells of sauropods, hadrosaurs, and potential basal Tetanurae while being conspicuously rare in those of maniraptorans (including birds), suggesting that the formation mechanism may have changed during the evolution of eggshells from non-avian theropods to modern birds.
From a macroevolutionary perspective, SEUs appear in turtles, crocodilians, as well as ornithischian, sauropod, and theropod dinosaur lineages, indicating that these structures may have evolved independently within the calcitic layers of the eggshells. However, at the molecular level of biomineralization, the possibility of a deep homology of SEUs across all these lineages cannot be ruled out.
This study, published in Science Advances, sheds light on the characterization of SEUs in dinosaur eggshells and offers new insights into the evolutionary plasticity of eggshell structures.
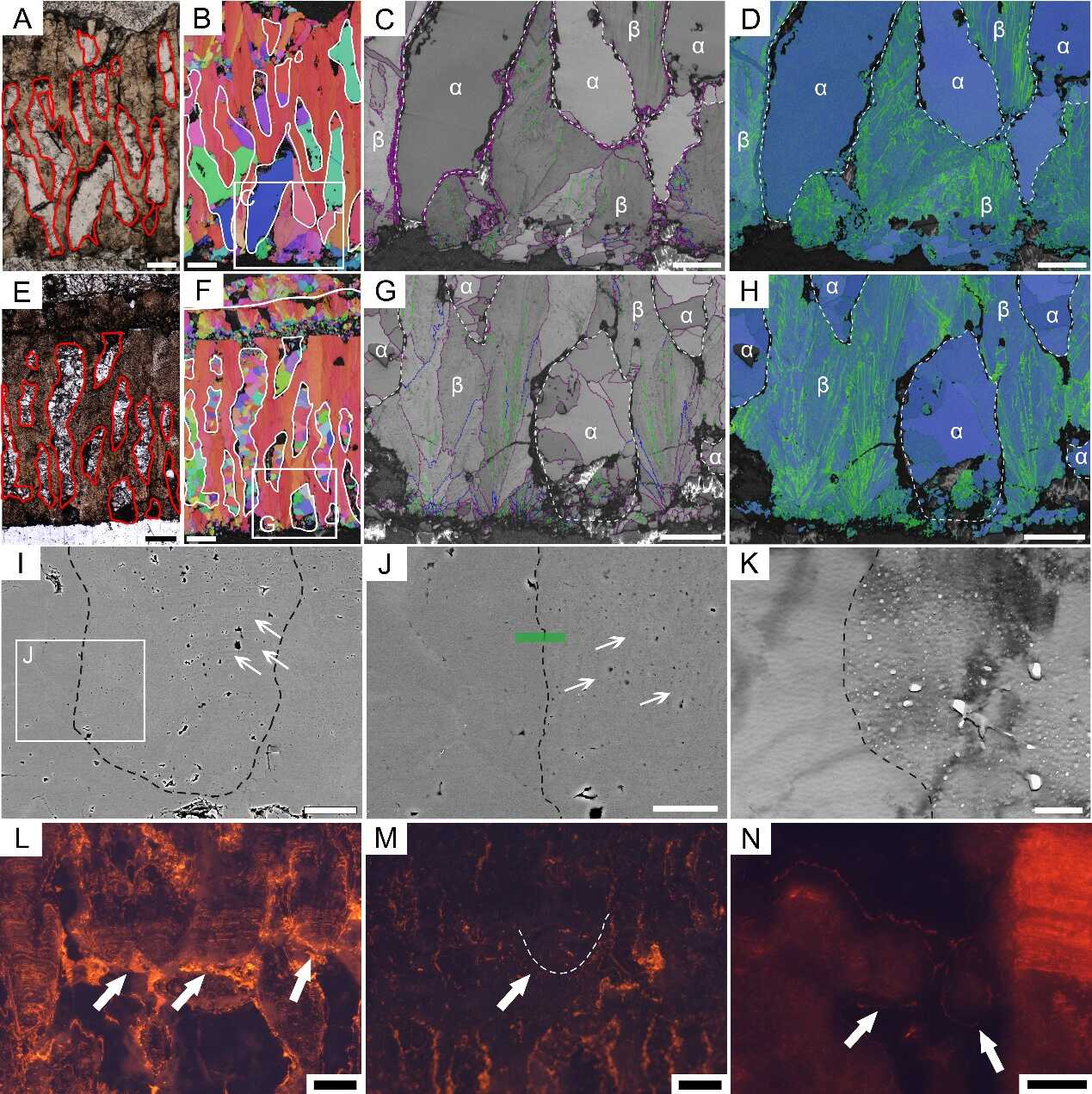
The differences between the biogenic and abiogenic calcite in dinosaur eggshells: In the PLM images (A and E), biogenic calcite is dark, while abiogenic calcite is bright; On the IPF maps (B and F), the directions of the c-axes of the biogenic calcite grains are consistent (red), while various in the abiogenic calcite grains (various colors); On the BC+GB maps (C and G), biogenic calcite has textured gray scales, while abiogenic calcite has plain gray colors; On the KAM maps (D and H) , biogenic calcite has high KAM values (green), while abiogenic calcite has low KAM values (blue). SEM (I and J) and TEM (K) images show that biogenic calcite has growth lines (arrows) and porous structure. In the CL images (L—N), SEUs are dark or have orange fluorescence, indicating that CL is not an effective method for the identification of biogenic calcite. Scale bars are equal to 200 μm (A, B, E, and F), 100 μm (C, D, G, H, and N), 50 μm (I),15 μm (J), 500 nm (K), and 150 μm (L and M). (Image by IVPP)
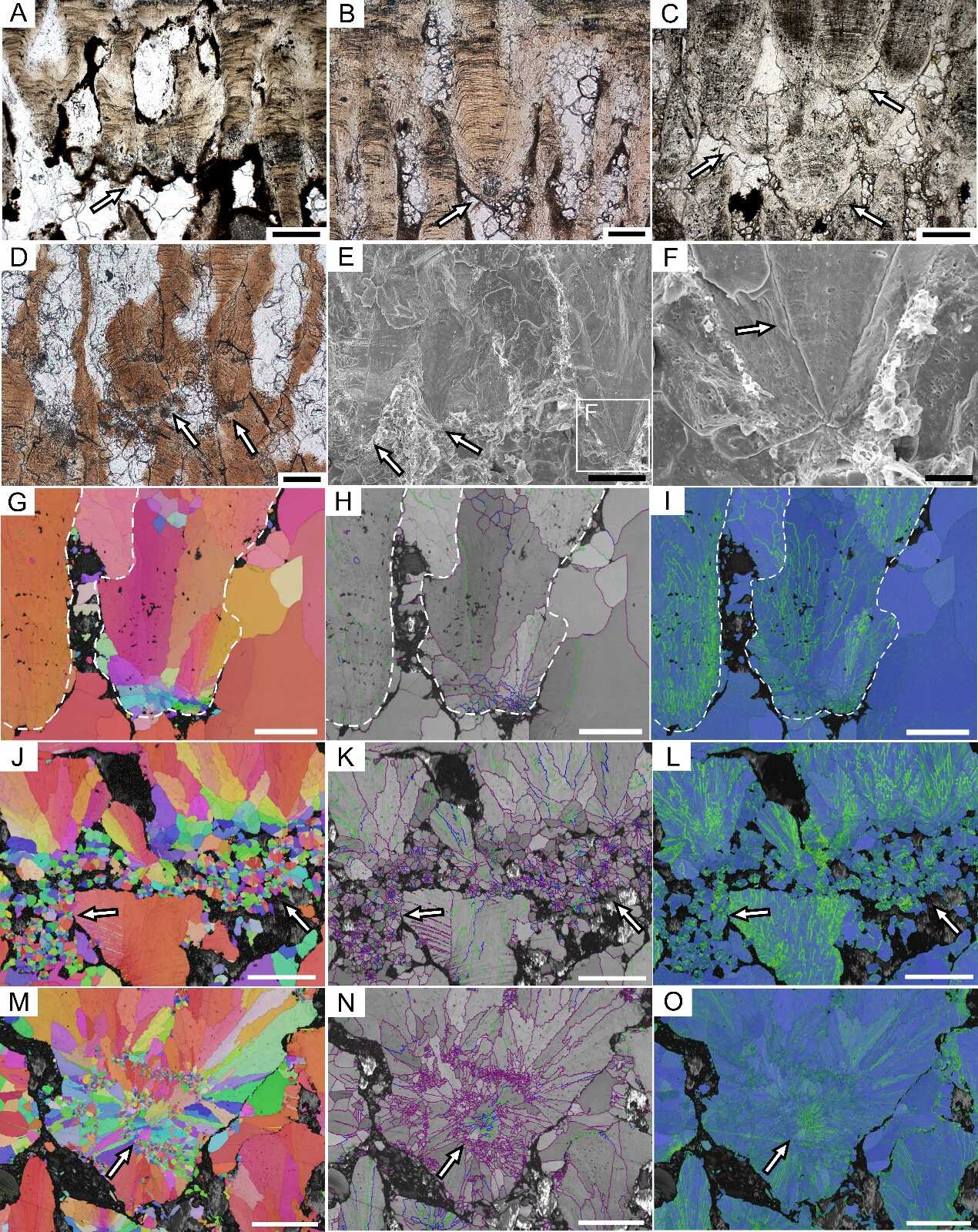
SEUs of the highly porous dinosaur eggshells. A—D: PLM images;E, F: SEM images;G—O: EBSD images. The SEUs have radially arranged c-axes (G, J, and M), textured gray scales (H, K, and N), and high KAM values (I, L, and O). The arrows show the grooves and vesicles in F, show the SEUs in other images. Scale bars are equal to 200 μm (A and C), 100 μm (B, D, E, and G to O), and 25 μm (F). (Image by IVPP)
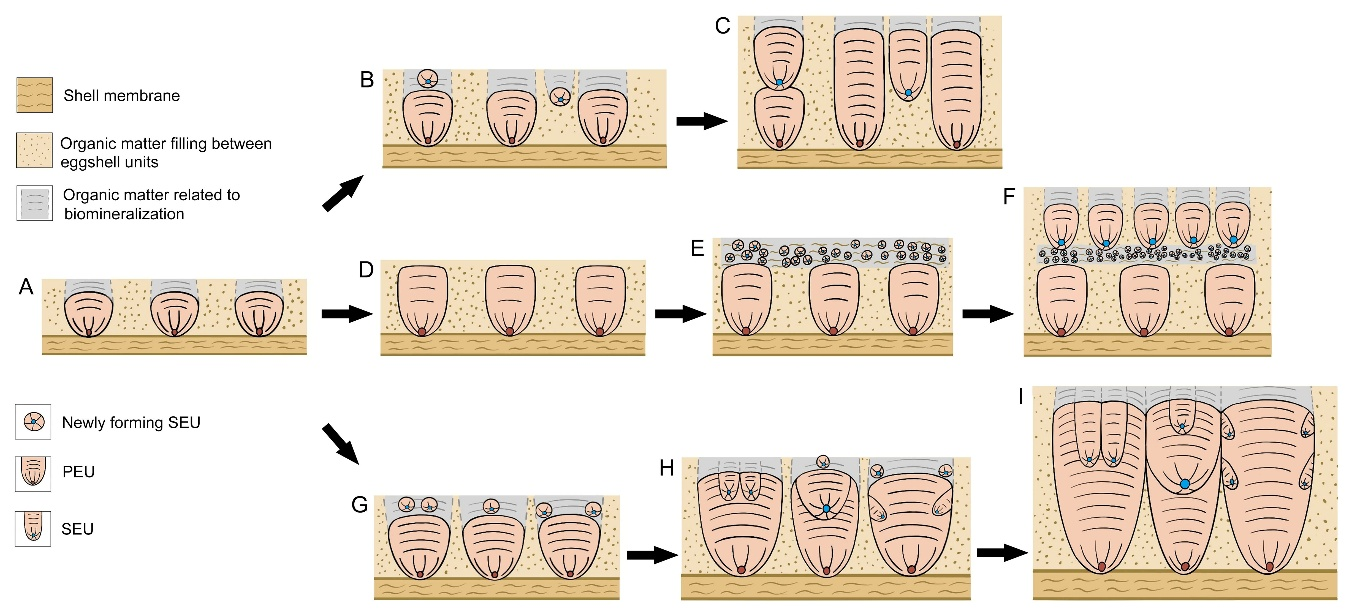
Formation mechanism of dinosaur eggshells containing SEUs. B—F: Highly porous eggshells, SEUs are superimposed on PEUs, inside pore canals (B and C), and form layers (E and F);G—I: Moderately porous eggshells. SEUs are mostly embedded in PEUs and other SEUs. (Image by IVPP)
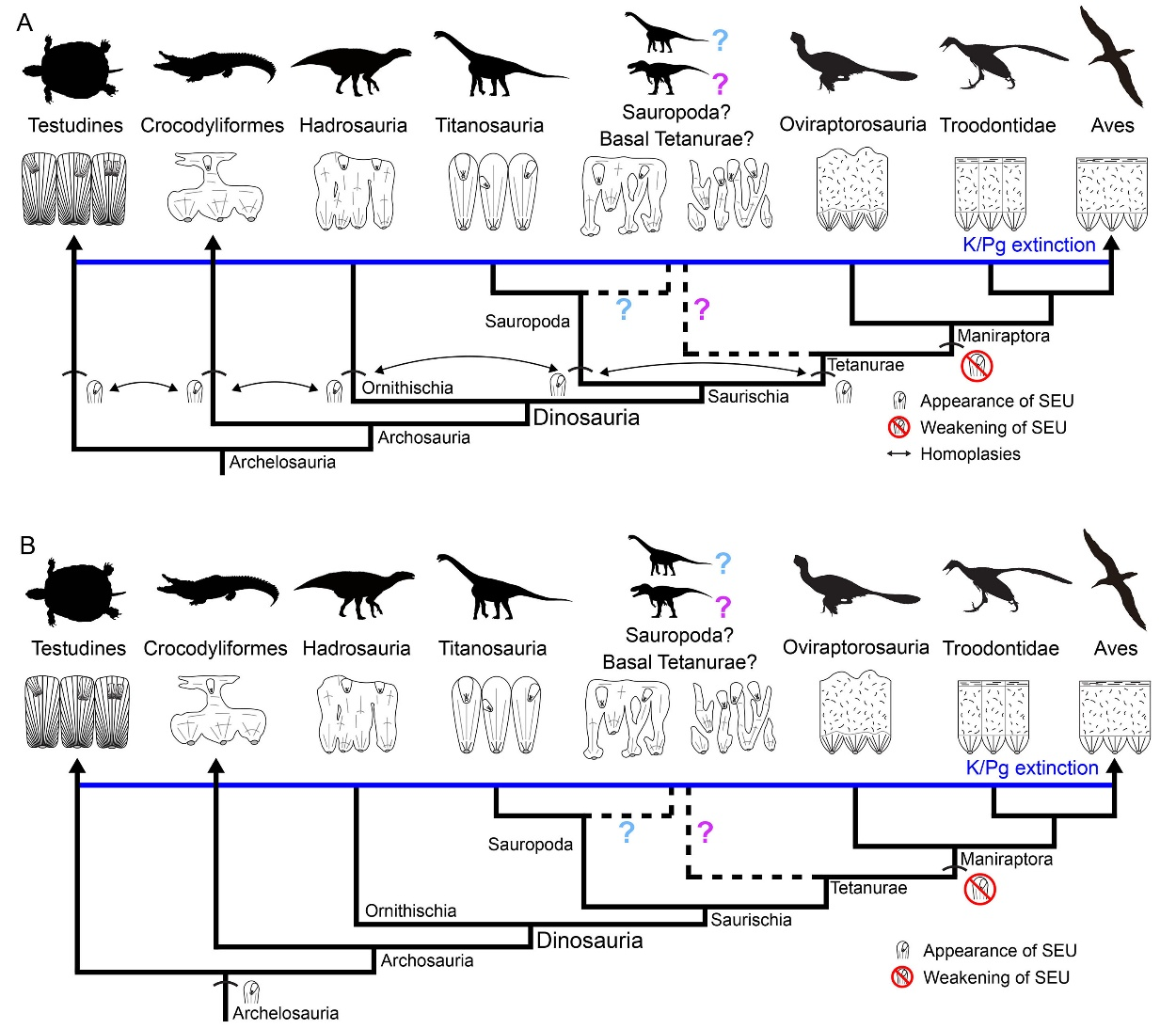
SEUs gradually decline in the maniraptoran eggshells. The SEUs of turtle, crocodilian, ornithischian dinosaurs, sauropod dinosaurs, and theropod dinosaurs might result from parallel evolution (A), or might have a deep homology (B). (Image by IVPP)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

